Preparations
- Prepare sodium acetate
Distal water: 200 ml – Sodium acetate 61.525g – Adjust pH to 5.2 – Add distilled water to reach 250ml – Filter solution
- LB+AGARE plate with ampicillin
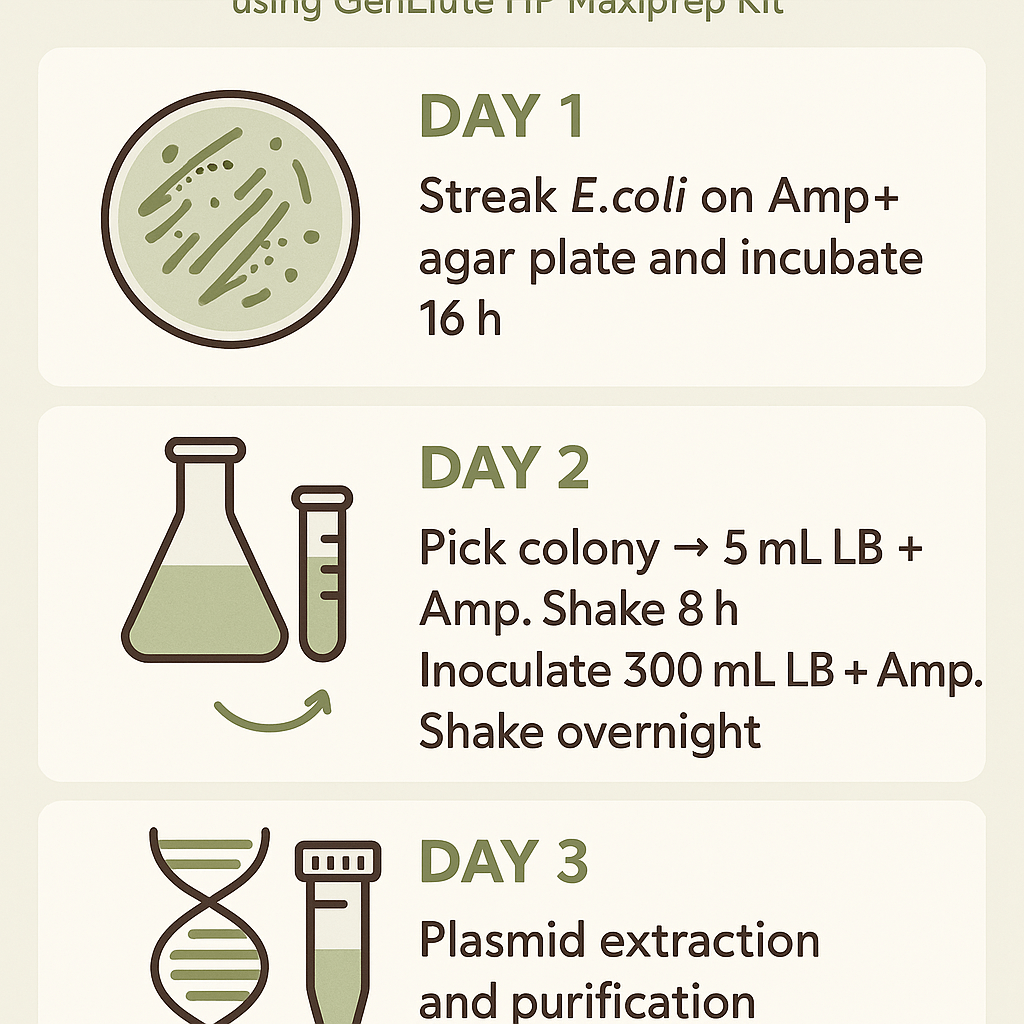
Day 1:
- Streak agar plate containing ampicillin with E. coli containing the desired DNA.
- Put the plate in the incubator for 16 h
Day 2:
- Take the plate out of the incubator.
- Take one colony and inoculate it in a starter culture containing 5 mL LB + 5 μL Ampicillin
- Put in a shaking incubator for 8 h.
- After 8h, mix the culture with a pipette, then take 300 μL of starter culture and add it to the pre-prepared 300 ml LB culture that was supplemented with 300ul ampicillin.
- Put in the incubator for 14 h at 250 rbm
Day 3:
- Measure the OD by adding 500 ul LB to the cuvette as blank
- Add 450 μL LB to cuvette +50 μL from the culture mix.
- Measure in the spectrophotometer if it’s 0.3 its good
Then we proceed to the next step, following the (GenEluteTM HP Plasmid Maxiprep Kit )
- Harvesting cells:
Pellet 300 ml of overnight culture by centrifugation at 5000 xg for 10 min and discard supernatant.
- Resuspend cells: add 12 ml of resuspension solution, then vortex until it is melted
- Lyse cells: add 12 ml of lyse solution, then mix by inverting 8 times, let it sit for 5 min.
- Neutralize: add 12 mL of chilled neutralization solution and mix 6 times.
- Add 9 mL binding solution and invert 2 times, let sit for 5 min.
- Add 12ml of column binding solution to the column and spin in a swinging bucket at 3000xg for 2 min.
- Hold filter barrel over binding column and gently apply pressure to plunger, expelling half of the cleared lysate into the column. be careful not to overfill. Spin in the swinging bucket at 3000xg for 2 min. Discard the elute. Add the rest of the cleared lysate and repeat the spin.
- Apply wash solution 1, spin in a swinging bucket at 3000xg for 2 min. Discard the elute.
- Apply wash solution 2, spin in swinging bucket at 3000xg for 5 min. discard elute.
- Dray spin at 3000xg for 5 min.
- Elute the plasmid: add the column to a new centrifuge tube, then add 3ml elution solution.
- Leave in room temperature for 5 min.
- Spin in the swinging bucket at 1000xg for 5 min.
- DNA Concentration: Add 0.1 volume of 3M Sodium acetate and 0.7 volume of isopropanol to the recovered plasmid. Mix well by inversion and centrifuge at 10000xg for 45 min
- Decent supernatant, add ethanol 70% 1.5 ml, mix well.
- Distribute in an Eppendorf tube—centrifuge at 10000xg for 15 min.
- Air dray 30 min