expiCHO stand for chinese hamster ovary and its an epithelial cell line with several advantges for biopharma, they are commonly used in recombinant protein production as well as in scientific research
Thawing and passaging expiCHO-S cells
Materials :
- Erlmnmeyr Flask 125 ml
- CD optiCHO medium
- expiCHO-S cell vial
- gun
- serological pipette
Day 1:
- Warm CD optiCHO media in incubator .
- After the media is warm Thaw 1 vail of cells in water bath for 30 sec then spray the vial with 70% ethanol.
- open the vial in a class II biological cabinet.
- Add 30 or 35 ml media ( depend on your need ) to the flask.
- Take 1ml from the media in the flask and add it to the vial to thaw the left over ice.
- Remov all the cell content from the vial and add it to the flask.
Close the flask and put it in a shaking incubator 37°C , 8% CO2 Orbital shaker platform:
• 125 ±5 rpm (19-mm shaker throw)
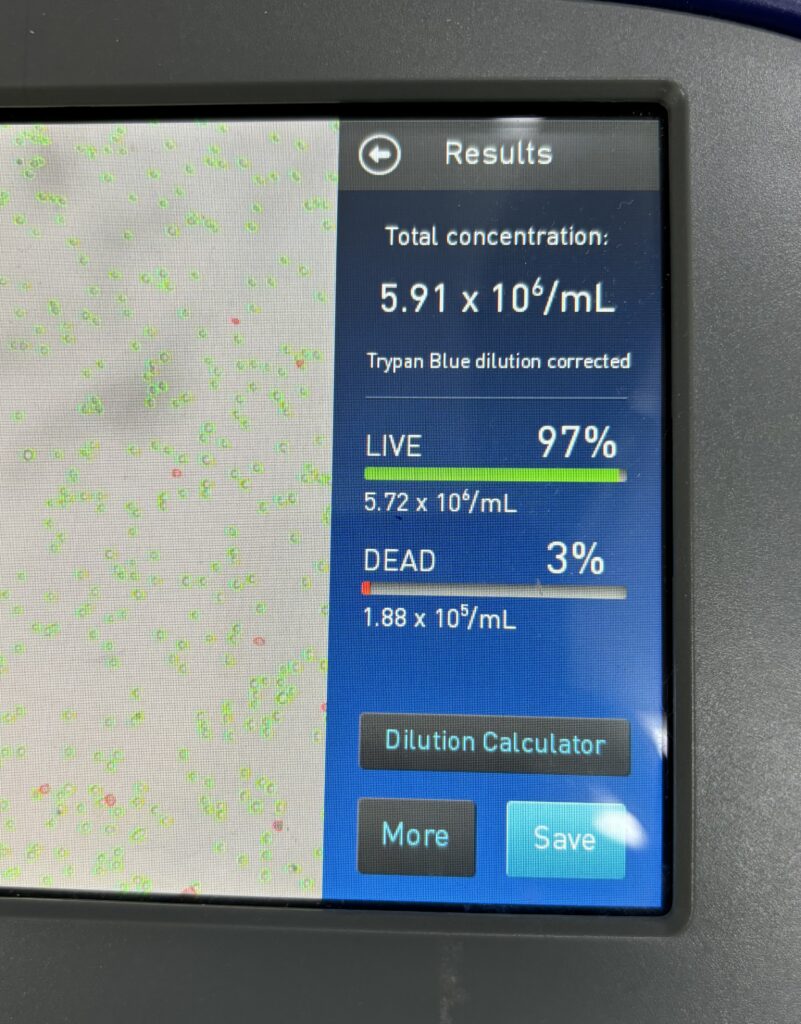
Day 3-4: count cells and determine viability
Materials:
- hemocytometer
- trypan blue
- Take the flask out in the biocabinet, take a very small amount in an Eppendorf tube. Rutern the flask to the incubater.
- You must work quickly and fast, add 10 μL from the Eppendorf to another tube and add 10 μL of trypan blue, mix, and add to the hemocytometer, then read it in counts.
- Cell viability should be ≥95%
- You can split the cells at this stage.
Transfections of 40 mL starter volume
- Set up 250 mL flask with 60 mL ExpiCHO-S cells at 2.5-3 x106 cells/mLDay (-1):One day prior transfection Seed at 2.8-3 x106 cells/mL in 250 mL flasks with 40 mL ExpiCHO-S in (CD opti CHO media)
- Cells were 2.6 x106 cells/mL, V > 97%, at 9:00
- Day (0):
- On the transfection day, cells should be 5-6 x106 cells/mL, V > 95%.
- Cells were 6 or5 x106 cells/mL, V > 98%, at 8:00
- On day of transfection, dilute cells using CD CHO media , to a final density 6 x106 cells/mL. Viability should be 95–99% to proceed with transfection.
- 30% of the starter volume (40 mL) of OptiPro SFM = 12 mL –> ( .3×40=12 SFM )
- 0.5 µg DNA/1 million cells/1 mL cells = 0.5 × 5 × 40 = 100 µg
- PEI to DNA ratio 3:1= 3 × 100 = 300 µL
- All transfectants:
- Dilute cultures 1:2 (v:v) CALCULATIONS ( 12+40=52×2=104 mL) with 8 mM GlutaMax CD OptiCHO (80 mL) supplemented with final culture concentrations of 7.5% (v:v) Efficient Feed A (11.7 mL), 7.5% (v:v) Efficient Feed B (11.7 mL).
- ACA 0.4% of the total volume (.624 mL) = 624 µL
For each construct do the following:
- Set up DNA mix: Total of 100 µg DNA + 6 ml OptiPro SFM.
- Set up PEIpro mix: 300 µL PEIpro + 6 mL OptiPro SFM
- Combine DNA and PEIpro mixes in 50 mL falcon tube approx., 10 mins incubation at room temperature without shaking. (N.B. Add the PEIpro/OptiPro mix onto the DNA/PEIpro mix, not the other way around)
- Add 32 mL DNA/PEIpro to 100 mL cells ~4 – 6 hrs at 37C.
- Add 260 mL Feed:
- 11.7 mL Feed A
- 11.7 mL Feed B
- 624 µL Anti clumping Agent
- 80 mL CD OptiCHO + 8mM GlutaMax
- Gives total volume of 156 mL, which will be seeded in:
- 500 mL flasks (96 mL).
- 250 mL flasks (60 mL).
- Incubate flasks at mild hypothermic condition, 32C, 6% CO2
- Harvesting:
- Harvest, then centrifuge the supernatant at 3500 ×g for 15 minutes in a refrigerated centrifuge.
- Then, centrifuge the supernatant at 8000 ×g for 15 minutes in a refrigerated centrifuge.
- Filter supernatant through a 0.22μm filter.
Planing
Thawing – passaging -passaging (because you need to passage 3 times before Transfections)
Upscaling( is a process of increasing the volume of the culture) – seeding (before you seed passag another 60 ml ) – Transfections. Repeat the process from the passage you made.